If all the money spent on military budgets in every country had been devoted to biological research, the question of immortality, or at least eternal youth, would already have been resolved. (translation). Jean Rostand. French biologist, died in 1977.
The Heales Journey: Celebrating 200 Editions of the Newsletter
Looking back on the first edition
As we reach the 200th edition of Death of the Death, it is worth briefly returning to the very first newsletter published in January 2009. Issue number 0 introduced the ambition of following scientific progress related to human longevity, with a focus on the possibility of delaying, and potentially overcoming, age-related mortality.
The newsletter presented the concept of longevity escape velocity, the hypothesis that if advances in biomedicine increase life expectancy faster than time subtracts it, each generation of progress could enable the next. At the time, this idea was emerging in research circles, and the newsletter aimed to make it accessible and track developments in fields such as regeneration, stem cells and aging mechanisms.
Sixteen years later, this 200th issue marks continuity rather than conclusion. We know more, we live on average longer, but maximal lifespan did not extend. The same questions remain open, the same scientific domains continue to evolve, and the initial objective persists: documenting the progress, challenges and perspectives of longevity science over time.
For this newsletter, we give you 200 pieces of information about longevity and about our organization. They are regrouped into 16 categories. It is impossible to be complete and objective, but we tried.
Top Geroscience Scientist / Personalities
Famous people who lived 100+ years
Organizations for longevity
Heales at important conferences
Conferences from Heales
Heales in the Media
Activities supported by Heales
Sport & Exercise Related to Longevity
Foods that could help longevity
Social Factors That Support Longevity
Biomarkers of Longevity
Genes Related to Longevity
Longevity products
Lesser-known facts in aging research
Bad News (Long way to go)
Discoveries and Technologies
Top Geroscience Scientist / Personalities
- Nir Barzilai. Medical Doctor Geneticist focused on aging. longevity genes. and interventions like metformin (Institute for Aging Research). Advocate of the TAME project.
- Irina Conboy. Her heterochronic parabiosis and plasma dilution studies revealed how systemic factors regulate aging and repair.
- José Cordeiro. Futurist and transhumanist author advocating radical life extension and the end of involuntary aging.
- Aubrey de Grey. Biomedical gerontologist and revitalization biotechnology advocate (LEV Foundation).
- Greg Fahy. Led human thymus regeneration studies (TRIIM), a landmark immunological aging trial.
- Steven Horvath. Creator of the epigenetic clock, one of the most influential biomarkers in modern aging biology. His DNA methylation clocks are used globally to measure biological age and evaluate rejuvenation interventions.
- Bryan Johnson. Entrepreneur running the Blueprint Project. an extreme data-driven experiment to slow and reverse biological aging in humans.
- Brian Kennedy. Distinguished professor in healthy longevity and biochemistry; long-time leader in aging biology.
- Cynthia Kenyon. Molecular biologist whose work with C. elegans revolutionized the genetics of aging.
- James L. Kirkland. Director of the Mayo Clinic’s Robert and Arlene Kogod Center on Aging, pioneered senolytics and demonstrated that clearing senescent cells improves healthspan. His work helped establish dasatinib and quercetin as the first-generation senolytic compounds.
- Andrea Maier. Prominent longevity medicine clinician and advocate for equitable geroscience translation.
- João Pedro de Magalhães. A leading computational geroscientist known for longevity genomics, comparative biology, and building the Human Ageing Genomic Resources (HAGR). His work spans AI-based drug discovery and the evolution of lifespan across species.
- Élie (Ilya) Metchnikoff. (†) Often widely credited as the father of gerontology. He coined the term “gerontology” in 1903 to describe the emerging scientific study of aging and longevity. He was a Nobel Prize winner in 1908 for his work on immunity, and devoted his later research to the concept of human longevity. His work laid the groundwork for modern aging studies and centered on the hypothesis that aging was a result of chronic auto-intoxication by gut bacteria.
- Liz Parrish. CEO of BioViva, known for pioneering the first self-administered gene therapy experiments aimed at reversing aging.
- David Sinclair. Harvard biologist and popular author on mechanisms of aging (e.g., sirtuins/NAD pathways).
- Shinya Yamanaka. Nobel Prize winning stem cell researcher who discovered induced pluripotent stem cells (iPSCs), foundational to cellular reprogramming and rejuvenation research.
- Alex Zhavoronkov. Founder and CEO of Insilico Medicine, is a leading figure in AI-driven drug discovery and computational geroscience. His work includes developing deep-learning-based aging clocks and multi-omics biomarkers for biological age.
Famous people who lived 100+ years
18. Jeanne Calment (122) (†). Oldest woman ever.
19. Jiroemon Kimura (116) (†). Oldest man ever.
20. Kane Tanaka (119) (†)
21. Sarah Knauss (119) (†)
22. Terentia (103) (†). Roman Empire. Widow of Cicero.
23. Edgar Morin (104). Oldest well-known philosopher.
24. Kirk Douglas (103) (†).
Organizations for longevity
25. Google Calico. Focusing on both basic research and the translation of our discoveries into new interventions that can help people live healthier, and maybe longer, lives.
26. Chan Zuckerberg Initiative (not “officially” longevity). It was founded in 2015 to help solve some of society’s toughest challenges — from eradicating disease and improving education to addressing the needs of our local communities.
27. Altos Labs. Restore cell health and resilience through cellular rejuvenation programming to reverse disease, injury, and disabilities that can occur throughout life.
28. BioViva Science (Liz Parrish). BioViva is committed to lengthening healthy human lifespans with AAV and CMV gene therapy (works with Integrated Health Systems.)
29. Longevity Escape Velocity Foundation (Aubrey de Grey). Exists to proactively identify and address the most challenging obstacles on the path to the widespread availability of genuinely effective treatments to prevent and reverse human age-related disease.
30. Rejuvenate Bio (George Church). Will make dogs (and later humans) “younger” by adding new DNA instructions to their bodies.
31. Dog Aging Project. The goal of the Dog Aging Project is to understand how genes, lifestyle, and environment influence aging. We want to use that information to help people increase their healthspan, the period of life spent free from disease.
32. National Institute of Aging (USA). Lead a broad scientific effort to understand the nature of aging and to extend healthy, active years of life. The Interventions Testing Program (ITP) is a peer-reviewed program designed to identify agents that extend the lifespan and health span in mice.
33. Institut Pasteur de Lille, Founded in 2003 by Prof Miroslav Radman and Prof Marija Alačević, is a research center, which mobilizes 34 research teams and aims to decipher the essential physiopathological mechanisms of the most impacting diseases, particularly infectious ones, to understand these diseases, slow down their development and imagine the treatments of tomorrow.
34. Salk Institute (Juan Carlos Izpisua Belmonte). The Institute is an independent nonprofit organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge. Be it cancer or Alzheimer’s, aging, or diabetes.
35. Buck Institute for Research on Aging, Mission is to end the threat of age-related disease for this and future generations.
36. Glenn Consortium for Research in Aging (11 centers). To extend the healthy years of life through research on mechanisms of biology that govern normal human aging and its related physiological decline, to translate research into interventions.
37. Life Biosciences (David Sinclair and Nir Barzilai). Research and development on therapeutics for human health. (See also Elixir Pharmaceuticals and Sirtris Pharmaceutical)
38. Longevity Research Institute (Joe Betts-Lacroix, Sarah Constantin, Jaan Tallinn). A health-span-expanding treatment for humans would prevent years of severe illness for billions of people. Plan to design, fund, and launch animal lifespan studies for the most promising longevity interventions.
39. Retro Biosciences. The mission is to add 10 years to a healthy human lifespan They are starting with cellular reprogramming, autophagy & plasma-inspired therapeutics.
40. International Longevity Alliance. Promotes longevity research and advocacy from the grassroots to the international level. It includes over 75 non-profit associations working in over 65 countries.
41. Hevolution. Funds efforts to extend healthy human lifespan and understand the processes of aging.
42. Lifespan Research Institute. Raising funds and awareness for scientific work addressing of the aging process and working directly on research projects.
43. XPrize Healthspan. Prize of $ 101 million for innovative therapies that restore muscle, cognitive, and immune function by a minimum of 10 years to make healthy aging possible for everyone.
Heales’ presence at certain conferences and activities
44. Web2Day. L’homme qui vivra 100 ans est déjà né. 2015.
45. TEDxULB “Eternal Life: Are We There Yet?”. TEDx talk of Didier Coeurnelle
2016, Université libre de Bruxelles (Belgium)
46. 2017. Longévité : un vieux rêve de l’humanité, peut-être le plus beau | Didier Coeurnelle | TEDxBelfort
47. “Afternoon of Study: Aging”. Invited speaker / discussant 9 December 2019, Brussels (healthy life expectancy; organized by the Belgian Federal Public Service Social Security)
48. Longevity Projects for Africa. Conference presentation 2019.
49. TransVision 2022, Paris, En route vers l’immortalité.
50. Longevity Summit Dublin. Speaker in 2022, 2023 and 2024
51. TransVision Utrecht 2024. Heales oral presentation
52. TransVision Abidjan 2025. Longévité, égalité, fraternité.
Conferences from Heales
53. The 1st Eurosymposium on Healthy Ageing (EHA) was held in 2012. The talks spanning 3 days included topics like- Biology of aging is now a robust science, and it can extend healthy lives, Concrete examples of research and innovation to extend healthy lives, and meeting among stakeholders: building the innovations together
54. The 2nd Eurosymposium on Healthy Ageing was held on 1st and 2nd October 2014.
55. The 3rd Eurosymposium on Healthy Ageing was held on 29th and 30th September and 1st October 2016
56. The 4th Eurosymposium on Healthy Ageing was held on 7th-9th November 2018
57. 5th Eurosymposium on Healthy Ageing, was held on October 1, 2020 on zoom. How to Significantly Extend Healthy Lifespan. It adopted a Declaration on Biomarkers and Clinical Tests.
58. February 11, 2021. Conference and workshops. Clarifying whether and to what degree the current anti-aging approaches work in mice or people.
59. Virtual Conference On Big Data, A.I. and Healthy Longevity. How to progress faster and better for all scientists? Thursday, September 9, 2021
60. 6th Eurosymposium on Healthy Ageing (EHA). This meeting was held online on Friday, 25th, and Saturday, 26th November 2022. It adopted a Declaration for Radical Healthspan Extension: After Covid times, rejuvenation times.
61. Sharing Health Data and AI Insights for Longevity in Europe and Around the World. The conference explored the latest advancements in AI and Big Data within the realm of longevity research. February 29th, 2024. It adopted a Declaration on Sharing Health Data and Using AI for Healthy Longevity.
62. 7th Eurosymposium on Healthy Ageing. Friday, November 22nd, and Saturday, November 23rd 2024, “Sharing Health Data and AI Insights for Longevity in Europe”
Heales in the Media
63. 2014: Sciences humaines: Immortalité : the opinion of Didier Coeurnelle
64. 2014: RTL Belgium, Controverses « Bientôt tous immortels ?” Didier Coeurnelle
65. 2017: Pour augmenter la longévité humaine. Didier Coeurnelle, Long Long Life
66. 2018: interview, Belgian radio RTBF.
67. 2018: Belgian TV, RTBF, interview with Aubrey de Grey and Didier Coeurnelle, among others.
68. 2025: Sven Bulterijs Dutch journal Interview on Organ-Specific Aging Clocks (Het Nieuwsblad)
69. 2025: Sven Bulterijs Interview on Life Extension Following Remarks by Putin and Xi (VRT News)
Activities supported by Heales
70. Leucadia Therapeutics. Ferret study related to Alzheimer disease. Theory that reduced aperture sizes due to ossification leads to behavioral and brain morphological changes.
71. A study on older rats. To test longevity after injecting plasma fraction with the working name ‘Elixir’ into old rats (6 experimental old rats + 6 control old rats). This experiment was under the direction of Professor Harold Katcher in Mumbai, in collaboration with Heales.
72. Another study on older rats. To test longevity after plasma transfusion of young rats (9 tested old rats + 8 control old rats). This experiment was under the direction of Professor Rodolfo Goya at the Institute of Biochemical Research in Argentina, in collaboration with Heales.
73. DataBeta Test Project to compare epigenetic markers after testing different supplements, trying different diets and exercise programs.
74. Project with Longeavus Technologies. Combination of Known and Putative Longevity Therapeutics for Radical Life Extension in Mice
75. LongevityGPT is an AI tool that uses domain-specific retrieval and advanced AI techniques to help answer longevity and genetics research questions by integrating scientific databases and improving the accuracy of biomedical information retrieval. The main scientist behind this project is Anton Kulaga.
76. Project with Nicolas Chernavsky replicate one of the experiments of Harold Katcher’s study, which demonstrated the rejuvenation of old rats using extracellular particles derived from young pigs’ blood plasma.
77. The Longevity Escape Velocity Foundation received a €200,000 donation from Didier Coeurnelle, with up to an additional €200,000 pledged contingent on matching gifts raised by October 31, 2024. The funding supported pre-study pilot work for the next phase of the Robust Mouse Rejuvenation project, as the first phase, which began in February 2023, has been completed.
Sport & Exercise Related to Longevity
78. Regular walking
79. Strength training
80. High-intensity interval training (HIIT)
81. Cycling
82. Swimming
83. Balance training
84. Flexibility / mobility work
85. Daily physical activity (non-exercise movement)
86. Cardiorespiratory fitness
87. Consistency over intensity
Foods that could help longevity
88. Olive oil (part of the Mediterranean diet)
89. Fatty fish (omega-3 rich) (part of the Okinawan diet)
90. Pomegranate
91. Nuts
92. Leafy green vegetables
93. Berries
94. Fermented foods (part of the Japanese diet)
95. Passion fruit
96. Green tea and coffee
97. Dark chocolate (high cocoa)
Social Factors That Support Longevity
98. Strong social ties
99. Regular social interaction
100. Believing in god
101. Intergenerational relationships
102. Being married or partnered (at least for men)
103. Cultural engagement
104. Feeling useful to others
Biomarkers of Longevity
105. Biological age (epigenetic age)
106. Resting heart rate
107. VO₂ max
108. Grip strength
109. Fasting glucose
110. HbA1c
111. Inflammatory markers (e.g. CRP)
112. LDL/HDL cholesterol ratio
113. Blood pressure
114. Muscle mass
Genes Related to Longevity
115. FOXO3
116. APOE
117. SIRT1
118. SIRT6
119. IGF-1 pathway genes
120. mTOR pathway genes
121. TP53
122. CETP
123. KLOTHO
124. LMNA
Longevity products:
125. NMN (Nicotinamide Mononucleotide) NAD⁺ precursor for cellular energy and aging support.
126. NR (Nicotinamide Riboside) another NAD⁺ precursor supporting mitochondrial health
127. Resveratrol polyphenol thought to activate longevity pathways.
128. Fisetin natural senolytic (clears senescent cells).
129. Quercetin antioxidant often paired with fisetin for senolysis.
130. Spermidine supports autophagy and cellular renewal.
131. Astaxanthin antioxidant with mitochondrial and anti-aging support.
132. Coenzyme Q10 (CoQ10) supports energy production and cardiovascular health.
133. Curcumin anti-inflammatory antioxidant.
134. Pterostilbene resveratrol-like antioxidant with higher bioavailability.
135. Rapamycin (Sirolimus) mTOR inhibitor shown to extend lifespan in animal studies
136. Metformin diabetes drug with potential longevity benefits.
137. Senolytic Drug Combinations (e.g., Dasatinib + Quercetin) targeted clearance of senescent cells.
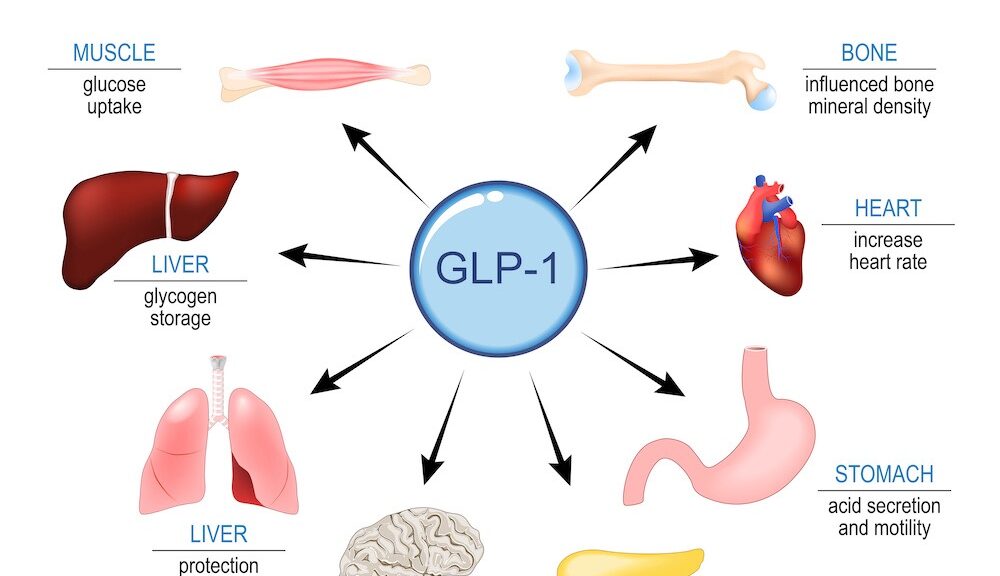
138. GLP-1 Receptor Agonists (e.g., semaglutide) diabetes/weight-loss drugs with potential systemic aging benefits.
139. SGLT2 Inhibitors cardio-renal protective drugs with possible longevity implications
140. Omega-3 Fatty Acids (fish oil DHA/EPA) cardiovascular and anti-inflammatory effects tied to healthy aging.
141. Vitamin D (plus Vitamin K2) supports bone health, immune function, and cellular longevity markers.
142. Alpha-Ketoglutarate (AKG) metabolic intermediate linked to reduced inflammation and energy metabolism support.
143. Longevity Complete™ Supplement Blends multi-ingredient commercial products combining NAD⁺ precursors, CoQ10, antioxidants, and other longevity agents.
Lesser-known facts in aging research
144. Sharks get cancer less often than other species, probably because of a slow mutation rate.
145. Some jellyfish can revert to their juvenile form repeatedly, essentially “aging backward.”
146. Sleep timing affects your lifespan people with irregular sleep patterns age faster.
147. Extreme cold exposure may trigger longevity pathways in humans and animals.
148. Certain protein-restricted diets without calorie reduction can extend lifespan.
149. Your skin age can differ drastically from your biological age internally.
150. Blue zones have unique social habits that may be as important as diet.
151. Some long-lived species tend to have very stable blood sugar levels naturally.
152. Telomere shortening isn’t the only clock of aging other protective DNA loops exist.
153. High cardiovascular fitness can add more years than diet alone.
154. Some long-lived rodents resist cancer almost completely.
155. Environmental enrichment can slow brain aging in mammals.
156. Certain RNA molecules may influence longevity independently of DNA.
157. Mitochondrial transplantation in lab animals can improve tissue function.
158. Some animals defy the “size vs lifespan” rule tiny bats can live 40+ years.
159. Some turtles can survive without oxygen for hours by slowing their metabolism dramatically.
160. Intermittent mild heat exposure (like sauna use) is linked to longer life in humans.
161. Long-lived whales accumulate fewer harmful mutations in their DNA over time.
162. Certain cavefish live longer than surface fish, despite harsh conditions.
163. Regular social interaction may protect telomeres and slow cellular aging. (More in exercise part)
Bad News (Long way to go)
164. No mouse in the world today is older than 4 years (and it was slightly better in the past).
165. No human is older than 116 years (and the oldest person ever, Jeanne Calment, lived 6 years more).
166. Pollution of microplastics is increasing fast and going in our brains. We do not know how to stop this.
167. To live longer, there is still nothing really better than what your parents told you.
168. Eroom’s law. Creating new drugs is becoming slower and more expensive.
169. During Covid times, life expectancy in the world decreased for the first time since 70 years, despite more money being used for health than ever.
170. Life expectancy in the US is stagnating despite the USA spending more money for health than any other country and having many of the best scientists inthe world.
171. The prospect for (biological) immortality appears to be close since more than 60 years, wrongly until now.
172. Life expectancy is not rising faster during the 21st century than during the 20th century.
173. Failure of Alzheimer drugs and therapies in humans is close from 100 %.
Discoveries and Technologies
174. mTOR inhibition extends lifespan (rapamycin effects across species)
175. Senescent cells drive aging and their removal improves healthspan
176. Senolytic drugs selectively eliminate senescent cells
177. Epigenetic clocks accurately measure biological age
178. Partial cellular reprogramming can reverse aging markers without loss of identity
179. Inflammaging identified as a central mechanism of age-related diseases
180. Mitochondrial dysfunction as a root cause of aging
181. Stem cell exhaustion recognized as a hallmark of aging
182. Gut microbiome influences aging and lifespan
183. Caloric restriction mimetics identified (e.g. metformin, rapamycin)
184. Proteostasis collapse linked to neurodegeneration and aging
185. DNA damage accumulation and repair decline tied to aging speed
186. Immune system aging (immunosenescence) mapped and quantified
187. Circulating “youthful” blood factors affect aging (heterochronic parabiosis)
188. Sex differences in aging biology formally characterized
189. Aging defined as a treatable biological process, not just a risk factor
190. Aging can be partially reversed by cellular reprogramming (Yamanaka factors)
191. CRISPR gene editing (and other gene therapies)
192. AI-driven drug discovery
193. Single-cell sequencing
194. Organoids
195. Wearable health trackers
196. Digital twins in medicine
197. Stem cell therapies
198. Advanced diagnostics (multi-omics)
199. Robotics for elderly care
The good news of the month.
200. We live longer than ever during the whole history of Humanity. On average, 73 years in the world. 85,5 years in Hong Kong.
For more information