« I predict one day it will be normal to go to a doctor and get a prescription for a medicine that will take you back a decade ». Sinclair said at a California event.
“There is no reason we couldn’t live 200 years.” David Sinclair, who runs an aging-research lab at Harvard University, says the new therapies could allow people to live much longer than they currently do.
This month’s theme: Neurodegenerative Diseases and Aging
Introduction
Among all diseases related to old age, Alzheimer’s disease is probably the most studied. Sadly, it is still also an incurable, very frequent disease.
Would all of us die of degenerative diseases if we were able to suppress all other causes of death related to aging? Probably, and this is not the funniest way to age and die (if there is one). And until now, all promising therapies have been globally unsuccessful even if they were promising discoveries to understand these diseases and even slow down the diseases on animal models.
We need more work, more clinical trials, and more imagination to progress in this domain.
Aging as a risk factor for neurodegenerative disease
The primary risk factor for most neurodegenerative diseases is aging, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). Most individuals with AD are aged ≥65 years and its prevalence continues to increase with increasing age. Tissues composed primarily of postmitotic cells, such as the brain, are especially sensitive to the effects of aging. The disease progresses irreversibly and is associated with high socioeconomic and personal costs. The nine biological hallmarks of aging are genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence, deregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication.
Aging is the main risk factor for most neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease.
This Cognitive Trajectories and Resilience in Centenarians study was done on 340 self-reported cognitively intact centenarians. Forty-four of these participants went on to neuropathological study and testing was performed with a range for the sample of 0 to 4 years.
There are some important findings from this work. During 1.6 years of follow-up, no decline in cognitive function was observed except for a minor decrement in memory. This suggests that, among this sample of centenarians, the incidence of dementia was low and implies resilience or resistance to AD and related dementias, despite the facts that they have the most potent risk factor in the general population, extreme old age, and that brain amyloid-β and tau deposition generally increase with age.
Various studies support the hypothesis that centenarians benefit from protective mechanisms rather than enjoying a relative absence of neurodegenerative causative factors.
Alzheimer plaques and tau proteins …
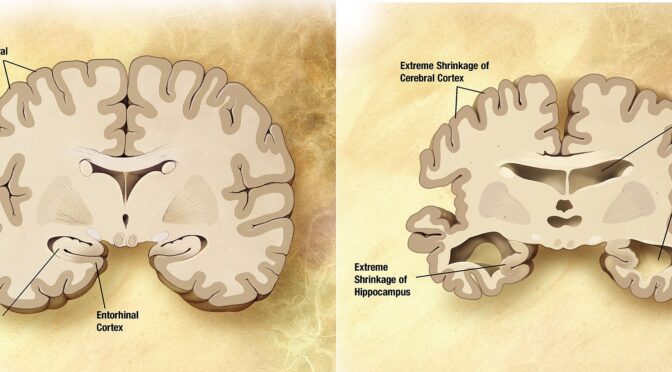
Alzheimer’s disease disrupts transmit information via electrical and chemical signals. among neurons, resulting in loss of function. Damage is widespread, as many neurons stop functioning, lose connections with other neurons, and die. Alzheimer’s disrupts processes vital to neurons and their networks, including communication, metabolism, and repair. The beta-amyloid protein involved comes in several different molecular forms that collect between neurons. Proteins clump together to form plaques.
Neurofibrillary tangles are abnormal accumulations of a protein called tau that collects inside neurons. In healthy neurons, tau normally binds to and stabilizes microtubules. In Alzheimer’s disease, however, abnormal chemical changes cause tau to detach from microtubules and stick to other tau molecules, forming threads that eventually join to form tangles inside neurons. It appears that abnormal tau accumulates in specific brain regions involved in memory. Beta-amyloid clumps into plaques between neurons. As the level of beta-amyloid reaches a tipping point, there is a rapid spread of tau throughout the brain.
Tests on mice are promising but never confirmed
One difficulty is the clear inability of current animal models to represent the full range of events identified in human disease, for instance, neuronal loss. It should be noted that a recent report using a Drosophila model suggests that neuronal loss may be protective in AD. This opens the door to a novel hypothesis that if proven would be quite atypical as in other neurodegenerative conditions, e.g. Parkinson’s and Huntington’s diseases, where neuronal loss is the main neuropathological feature.
A new mouse model developed by RIKEN researchers could improve the situation that many compounds that showed promise in mice models of the disease subsequently flopped in clinical trials on people. Because they so rapidly developed the signature brain abnormalities associated with Alzheimer’s disease, the mice should allow researchers to efficiently screen disease-modifying therapeutic candidates.
Do women have more often the disease?
Women living longer than men are probably not the whole answer as to why women are more likely than men to develop the disease. Your chances of developing Alzheimer’s disease late in life are somewhat greater if you are a woman than a man. One study followed 16,926 people in Sweden and found that beginning around age 80, women were more likely to be diagnosed than men of the same age. And a meta-analysis examining the incidence of the disease in Europe found that approximately 13 women out of 1,000 developed Alzheimer’s every year, compared to only 7 men.
A possible reason:
- The amyloid plaques that cause Alzheimer’s disease may be part of the brain’s immune system to fight against infections.
- Women have stronger immune systems than men.
- As part of their stronger immune systems, women may end up having more amyloid plaques than men.
Notably, mitochondria from young women are protected against amyloid-beta toxicity, generate less reactive oxygen species, and release fewer apoptogenic signals than those from men. However, all this advantage is lost in mitochondria from old females. Since estrogenic compounds protect against mitochondrial toxicity of amyloid-beta, estrogenic action, suggests a possible treatment or prevention strategy for AD
Possible therapies
Transplanted stem cells have shown their inherent advantages in improving cognitive impairment and memory dysfunction, although certain weaknesses or limitations need to be overcome.
The transplanted neural stem cells compensate for the loss of neurons and have a direct effect on the recipient tissue. Moreover, these cells can produce paracrine cytokines to exert an indirect effect on neurogenesis. The function of transplanted cells can be enhanced through preconditioning. For instance, the transplantation of transplanted neural stem cells that express growth factors promotes neurogenesis and improves cognitive impairment can ameliorate spatial memory and slow learning deficits. However, the transplanted cells can also transdifferentiate into non‐neuronal glia, which is an adverse event.
Organoids
Human neurodegenerative diseases, such as Alzheimer’s disease are not easily modeled in vitro due to the inaccessibility of brain tissue and the level of complexity required by existing cell culture systems. Three-dimensional brain organoid systems generated from human pluripotent stem cells have demonstrated considerable potential in recapitulating key features of AD pathophysiology, such as amyloid plaque- and neurofibrillary tangle-like structures. However, they fail to model complex cell-cell interactions of different regions of the human brain and aspects of natural processes such as cell differentiation and aging.
First-in-Human Clinical Trial to Assess Gene Therapy for Alzheimer’s Disease
Researchers at the University of California San Diego School of Medicine have launched a first-in-human Phase I clinical trial to assess the safety and efficacy of a gene therapy to deliver a key protein into the brains of persons with Alzheimer’s disease or Mild Cognitive Impairment, a condition that often precedes full-blown dementia.
The protein, called the neurotrophic factor is part of a family of growth factors found in the brain and central nervous system that support the survival of existing neurons and promote the growth and differentiation of new neurons and synapses. This is particularly important in brain regions susceptible to degeneration in AD.
Deep brain stimulation for Parkinson’s
For people with Parkinson’s disease who do not respond well to medications, the doctor may recommend deep brain stimulation. During a surgical procedure, a doctor implants electrodes into part of the brain and connects them to a small electrical device implanted in the chest. The device and electrodes painlessly stimulate specific areas in the brain that control movement in a way that may help stop many of the movement-related symptoms of Parkinson’s, such as tremors, slowness of movement, and rigidity. This works, unfortunately only for a certain time.
Conclusion
We know more about neurodegenerative diseases and especially Alzheimer’s Disease than about other diseases that we can cure. However, we still ignore and must find answers to fundamental questions:
- What is really starting the disease?
- What is precisely accelerating the disease?
- Are the accumulation of tau proteins and amyloid proteins the cause or the consequence of the diseases (the answer is probably « both », but to what extent?)?
- And of course, what are the working therapies to stop or at least slow down the disease
Good News of the month: Longest living (Sprague-Dawley strain) rat called Sima is 47 months old and is still alive.
Therapeutic that mimics young plasma could signpost the way to longevity wrote the Longevity Technology. The last oldest rat before this experiment died at 45.5 months and was under calorie deficit intervention, the one in the current experiment has therefore already lived longer. The Guardian quotes the well-known scientist, Prof Steve Horvath: “I think the results are stunning, Some people will criticize the results due to the low sample size. One swallow does not make a summer. But I believe the results because several complementary studies support them.”
Heales sponsored the experiment by Harold Katcher and the startup Yuvan, where the product E5 is purified from younger animals and given to 24-month-old female rats for rejuvenation purposes.
For more information