The truth is, of course, that death should no more be considered an acceptable part of life than smallpox or polio, both of which we have managed to bring under control without denouncing ourselves as pretentious. Alan Harrington, The Immortalist. Source.
This month’s theme: Blood Brain Barrier and Aging
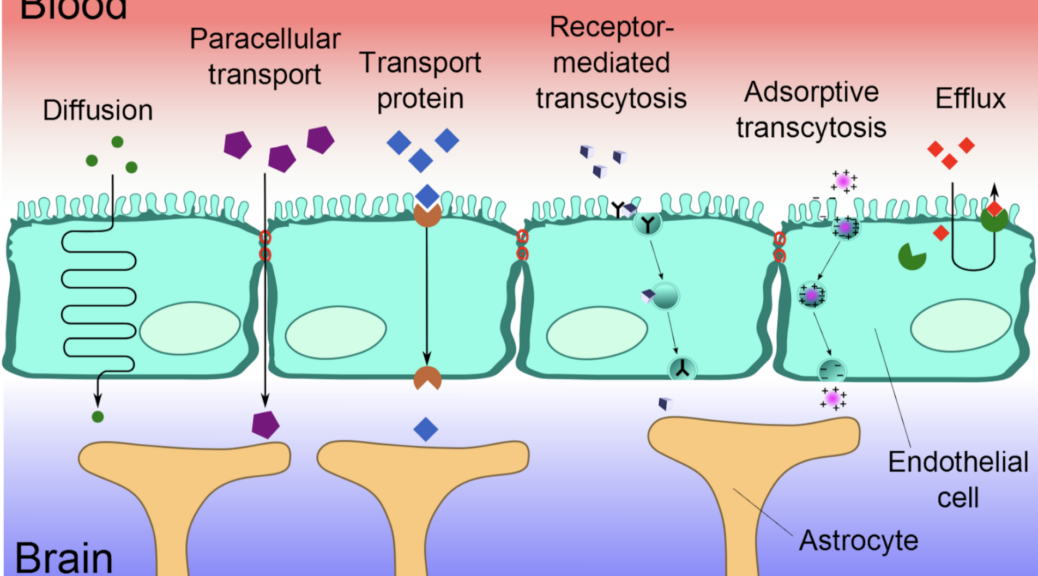
What is the Blood-Brain Barrier (BBB)?
The BBB is a highly selective barrier formed by endothelial cells of brain capillaries, supported by pericytes, astrocytes, and the extracellular matrix. It regulates the exchange of substances between the bloodstream and the brain, protecting the brain from toxins, pathogens, and inflammatory molecules, while allowing essential nutrients and gases to pass through.
BBB and Longevity
As we age, the BBB tends to become more permeable, leading to:
- Increased neuroinflammation: Leakiness of the BBB allows peripheral immune cells and inflammatory molecules to enter the brain, contributing to chronic neuroinflammation.
- Accumulation of toxins: Impaired BBB function leads to reduced clearance of neurotoxic substances like amyloid-beta, implicated in neurodegenerative diseases such as Alzheimer’s.
- Reduced nutrient transport: Nutrient and oxygen transport efficiency declines, affecting neuronal function and energy metabolism.
- Oxidative stress: Aging-related oxidative damage further compromises BBB integrity, exacerbating cognitive decline.
The aging process significantly impacts the blood-brain barrier (BBB), leading to functional decline that contributes to neurodegenerative diseases and cognitive impairment. Age-associated endothelial cell senescence disrupts tight junctions and increases BBB permeability, allowing harmful substances to infiltrate the brain and compromise its integrity. Senescent brain endothelial cells (BECs) also exhibit phenotypic alterations, including impaired tight junction regulation, exacerbating BBB dysfunction during aging.
Moreover, BBB decline varies by brain region and demographic factors, with studies showing a steeper decline in males than females in parietal and temporal areas as early as the 60s, likely due to sex-based protective mechanisms. Structural changes in BBB components, such as astrocytes and pericytes, further compromise its homeostasis, linking these alterations to neurodegenerative disease pathways. Increased BBB permeability due to vascular risk factors such as hypertension also directly correlates with white matter injury and cognitive decline, underscoring the importance of vascular health in mitigating these effects.
Maintaining BBB integrity is critical for cognitive health and overall longevity:
- Cognitive Reserve: Intact BBB function supports neural health, reducing the risk of age-related cognitive decline and dementia, major determinants of quality of life in older age.
- Neurovascular Coupling: Healthy BBB function supports optimal neurovascular coupling, which is essential for brain plasticity and repair mechanisms.
- Systemic Aging Impact: BBB dysfunction can lead to systemic inflammatory signaling, accelerating aging processes across other organ systems.
Several strategies show promise in maintaining BBB integrity and promoting longevity:
Exercise promotes vascular health, reduces inflammation, and enhances BBB integrity. Aerobic exercise has been shown to increase the expression of tight junction proteins and reduce oxidative stress in animal studies. The Mediterranean Diet is rich in antioxidants, omega-3 fatty acids, and polyphenols and this diet reduces oxidative stress and inflammation, protecting the BBB. Modest caloric restriction can reduce age-related BBB permeability by lowering systemic inflammation. Omega-3 Fatty Acids found in fish oil, enhance BBB integrity by reducing inflammation and promoting endothelial cell function. Found in berries, green tea, and dark chocolate, flavonoids protect against BBB dysfunction through their antioxidant properties. Vitamin E and C neutralize free radicals, protecting BBB endothelial cells from oxidative damage.
The brain-gut axis and the blood-brain barrier (BBB)
These are intricately linked systems that play crucial roles in maintaining both neurological and gastrointestinal health. The brain-gut axis is a bidirectional communication network involving the central nervous system, the enteric nervous system, the gut microbiota, and the immune and endocrine systems. This axis enables the brain and gut to influence each other through neural, hormonal, immune, and microbial pathways. The BBB, on the other hand, serves as a protective barrier that regulates the transport of substances between the bloodstream and the brain. This ensures that the central nervous system is shielded from toxins, pathogens, and fluctuations in blood chemistry while maintaining nutrient and signaling molecule access.
Disruptions in the brain-gut-BBB connection have significant implications for health and disease. Conditions such as neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s), mental health disorders (e.g., depression and anxiety), and autoimmune diseases (e.g., multiple sclerosis) are increasingly linked to dysfunction. Similarly, gut dysbiosis can exacerbate these conditions by altering neurotransmitter production, immune responses, and metabolic signaling.
The BBB is formed of specific cells. To protect the barrier from aging or even rejuvenate it, specific treatments that can be envisaged are treatments involving those cells.
Senolytics: A recent study investigated non-invasive biomarkers and their responses to a senolytic therapy combining dasatinib and quercetin (D + Q) in PS19 mice, a widely used tauopathy model. This study found D + Q treatment promoted a shift in microglial phenotype from a disease-associated state to a homeostatic state, reducing senescence-like features. Additionally, D + Q-treated PS19 mice showed improved cognitive performance in a tracing fear conditioning test, indicating enhanced cue-associated memory
mTOR Inhibitors: The results from a recent study identify mTOR activity as a key driver of BBB breakdown in Alzheimer’s disease (AD) and potentially in vascular cognitive impairment. They also suggest that rapamycin and related compounds (rapalogs) could serve as therapeutic agents for restoring BBB integrity in these conditions. This study highlights the mammalian/mechanistic target of rapamycin as a critical regulator of BBB breakdown in models of Alzheimer’s disease and vascular cognitive impairment. It underscores the potential of mTOR-targeting drugs to restore BBB integrity and mitigate disease progression.
Pituitary adenylate cyclase-activating polypeptide (PACAP): is a natural molecule with protective and growth-supporting effects on brain cells. Since PACAP and its receptor, PAC1, are found in brain regions affected by Alzheimer’s disease (AD), this study explores whether PACAP could be a helpful treatment for Alzheimer’s disease. A study tested PACAP in a mouse model of AD by giving it to the mice daily through their noses for an extended period. This treatment encouraged a healthier way of processing the amyloid precursor protein (APP), which reduced the production of harmful amyloid-beta (Aβ) proteins. It also increased the levels of brain-derived neurotrophic factor (BDNF), which supports brain health, and Bcl-2, a protein that prevents cell death.
Other Barriers of the Human Body
1. Physical Barriers
These act as the first line of defense to block the entry of harmful substances or organisms.
- Skin: A tough outer layer (stratum corneum) prevents the entry of pathogens and minimizes water loss. It acts as a mechanical shield.
- Mucous Membranes: Line body cavities (e.g., respiratory, digestive, and urogenital tracts). Produce mucus to trap microbes and particles.
- Tight Junctions: Found between epithelial cells in tissues like the gut and blood-brain barrier, preventing the passage of harmful substances.
2. Chemical Barriers
These involve substances produced by the body to neutralize or destroy pathogens.
- pH Levels: The acidic environment of the stomach (gastric acid, pH ~1.5–3.5) kills ingested pathogens. Skin and vaginal pH (slightly acidic) deter microbial growth.
- Enzymes: Lysozymes in saliva, tears, and mucus break down bacterial cell walls. Digestive enzymes (e.g., pepsin in the stomach) degrade microbial proteins.
- Antimicrobial Peptides: Defensins and cathelicidins disrupt microbial membranes and inhibit pathogen growth.
- Sweat and Sebum: Contain antimicrobial compounds and create an inhospitable environment for bacteria.
3. Biological Barriers
These involve living organisms or systems within the body that protect against pathogens.
- Microbiota (Flora): Commensal bacteria in the gut, skin, and other areas outcompete pathogens for resources and space. Produce substances (e.g., lactic acid) that inhibit harmful microbes.
- Immune Cells: Phagocytes (e.g., macrophages, neutrophils) engulf and destroy pathogens. Natural Killer (NK) cells target infected or abnormal cells.
4. Specialized Barriers
Certain structures serve as advanced protective mechanisms.
- Placental Barrier: Protects the fetus by regulating the exchange of nutrients, gases, and waste while preventing the passage of harmful substances.
- Corneal Barrier: Protects the eye, comprising a multi-layered structure (epithelium, stroma, and endothelium).
All those barriers, like the BBB, lose their efficiency when we age. This happens at different rhythms. The more we understand what is happening, the better our chances of finding new therapies. And in 2025, we still have much to discover concerning the diversity of the evolutions.
The good news of the month. Open discussion about (hereditary) genome editing.
An important article about gene therapy was written in Nature: We need to talk about human genome editing. « In a few decades, gene-editing technologies could reduce the likelihood of common human diseases. Societies must use this time to prepare for their arrival. Scientists know about tens of thousands of DNA variants that are associated with human diseases. On their own, the vast majority of these variants have small effects. But taken together, the result can be substantial. «
This point of view is opening the discussion about possible gene therapies for future generations. The diseases that we could cure are diseases that injure and kill mostly when people age since the mortality of young people is low, especially in rich countries.
For more information