Introduction
Aging (or senescence) has been the topic of many scientific research in recent years. Reaching an advanced age while remaining in good health was for a long time considered as a utopia. Nowadays, scientists are trying to analyze, understand and slow down the causes of aging.
The fight against aging is also a fight with our metabolism and the different causes that accelerate this process. In this text, we will explore the nine main causes of aging in humans, as described in a well-known article by scientists: Hallmarks of aging published in the journal Cell in 2013.
These nine causes have been subdivided into three subclasses: the primary causes, the antagonists of aging, and finally the secondary causes of aging that result from the first two.
Primary causes
- DNA damage
DNA is the carrier of the genetic material that confers certain specific characteristics to the human being. It is made up of a large number of nucleotides that serve to encode the genetic information of living beings into gene forms. The genome is the unique combination of a person’s genes, or genetic characteristics. It is located primarily in the nucleus of eukaryotic cells.
During cell division (mitosis), the genetic information contained in the mother cell will duplicate and be transmitted to each of the daughter cells, a process defined as DNA replication.
Certain life habits (cigarettes, food…) and other internal or external causes can affect the proper functioning of the genome over time, and thus accumulate errors in DNA replication. These errors can either repair themselves, or cause the death of the mother cell (apoptosis ) or be transmitted to the daughter cells.
Repair of DNA damage:
Our body contains certain genes that make proteins to repair the damage done during DNA replication. But the question is, “what happens to this repair system when our body reaches a certain age?”
The various coenzymes that participate in the repair mechanism of genome damage tend to decrease with age. Among them, NAD+. Several studies have been done in this field, with the aim of finding a correlation between our NAD+ level and the capacity of our organism to repair lesions in the DNA.
What is important to remember is that lesions in the DNA during replication can either lead to cell death or replicate defective cells. The accumulation of these lesions is one of the primary causes of aging.
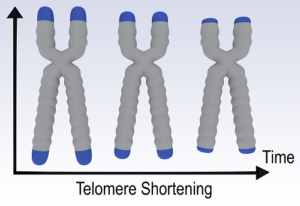
2. Telomere shrinkage
Telomeres are regions of DNA called “non-coding” (sequences of the genome that do not produce proteins), located at the end of each chromosome and whose main function is to protect the chromosomes.
During cell division, the DNA polymerase enzyme, responsible for DNA duplication, does not code the last nucleotides at the ends of the chromosomes, thus the telomeres. Thus, with each division, the telomeres shorten a little more until they disappear completely. With the disappearance of telomeres, the cell stops its division and dies. Some of our cells such as stem cells, germ cells and some of our somatic cells express an enzyme called telomerase. This enzyme is able to maintain the length of telomeres during cell division by adding a specific sequence of nucleotides to the ends of the chromosomes. Because of this mechanism, cells containing telomerase can replicate indefinitely.
Unfortunately, 90% of cancer cells express telomerase, which makes them capable of replicating forever as well.
The shrinking of telomeres causes our cells to die and our tissues to age. This implies an important place in anti-aging research.
3. Epigenetics
Epigenetics is the set of mechanisms that can modify the expression of genes without changing their DNA sequence.
Three main epigenetic modes of action that can influence lifespan have been identified:
– DNA methylation: a phenomenon that consists of adding or removing a methyl molecule in certain DNA sequences. The expression of genes can be modified by these changes which, by accumulating, can cause certain problems such as an increase in cholesterol levels or an increase in the risk of cardiovascular diseases.
– Histone acetylation: Histone acetylation and deacetylation are essential components of gene regulation. Acetylation, like methylation, is the process by which an acetyl functional group is transferred from one molecule to another. Deacetylation is the opposite, an acetyl group is removed from a molecule. These changes, too, can have some effect on our bodies and alter gene expression.
– Chromatin remodeling: Chromatin is the structure within which DNA is packaged. Chromatin can be remodeled, condensed. In its most condensed form it is called heterochromatin, in its least condensed form it is called euchromatin. An imbalance between heterochromatin and euchromatin can occur with age. This imbalance will change not only the stability of the chromosomes, but also the expression of the genes.
These epigenetic alterations can therefore modify gene expression and cause certain age-related diseases.
4. Protein folding
Proteins are present in every gene in our genome. The folding of proteins on themselves allows them to perform their multiple functions in our body. When protein folding is not done properly, it can be due to either a bad structure of the DNA or to a failure of the two recycling systems of the cellular building blocks. Both mechanisms deteriorate with age.
Misfolded proteins can accumulate in the body, even causing the death of some of our cells. This phenomenon is associated with many age-related diseases such as Parkinson’s disease, Alzheimer’s disease … It is therefore considered as one of the primary causes of aging.
The antagonists of aging
These are mechanisms that are initially supposed to protect us, but which eventually become harmful to the body.
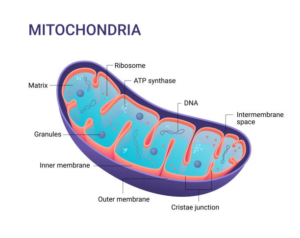
5. Mitochondrial dysfunction
Mitochondria are the powerhouses of cells. They maintain cellular respiration and contribute to the essential production of ATP (which provides energy). Mitochondria contain their own genes, the mitochondrial DNA. Abnormalities in these genes are associated with neurological disorders.
The mitochondria produce waste products, the free radicals. These free radicals by oxidation create damage to the mitochondrial DNA and proteins. The oxidative stress process triggers the autophagy mechanism to eliminate the damaged mitochondria.
The second cause that would link mitochondria to aging could be the communication between the nucleus of the cell and its mitochondria. With aging, these communications deteriorate.
6. Nutrient detection pathways
Our organism adapts the behavior of the cells according to the amount of nutrients available to them. Aging alters the process. Nutrient sensing pathways degrade. This can lead to atherosclerosis, the leading cause of cardiovascular disease, which is the leading cause of death in people over 70.
7. The senescence of cells
A senescent cell is an aging cell whose functions deteriorate. It stops dividing and its activity changes. During this change, a normal cell will begin to change its metabolism and may begin to secrete pro-inflammatory molecules that can, in turn, degrade the health of surrounding cells.
Senescent cells can be destroyed by the immune system. But this system decreases in efficiency with age. Therefore, aging causes senescent cells to accumulate, which in turn will degrade even more surrounding cells.
Secondary causes
9. Stem cell depletion
Stem cells are undifferentiated or partially differentiated cells. While normal cells are only able to divide a limited number of times (the so-called Hayflick limit), stem cells are able to divide in principle indefinitely.
However, with aging, the capacity of stem cells decreases, increasing the number of senescent cells present in the tissues and leading to various problems depending on the organ affected.
10. Inflammation
Inflammation is a complex phenomenon that occurs during an external or internal aggression on tissues. The cells of our body can emit signals according to the stress to which they are subjected. They synthesize molecules called cytokines to regulate the inflammatory response. There are also anti-inflammatory molecules that help regulate the inflammatory response. The goal is to achieve a balance so that the immune response is neither too low nor too high. With age, inflammation becomes more important and the regulations become less effective. This imbalance will cause what is called inflammagging.
Conclusion
The following describes the aspects of aging according to the most accepted categorization today.
There are many other theories related to aging, including the theory developed in Strategies for Engineering Negligible Senescence with not nine, but seven causes.
Does the extreme complexity of senescence mean that we cannot slow aging without addressing all aspects? No, because the causes of aging are very similar at least for many mammalian species. Opinions may vary on this question. However, these species have very different life spans. On the other hand, in order to achieve considerable progress, it is likely that all aspects of senescence will have to be taken into account.