Pessimism is about selling; optimism is about fighting. (…) If you look at life expectancy, you see that, around the 1850s, it was 30 years for women. Thirty years! Today, it is 85. She’s not the same woman anymore. It’s not the same body anymore. Michel Serres. Philosopher and science historian. Le Soir Monday, June 3, 2019, quoted following his death.
Theme of the month: The epigenetic clock, a marker of aging
A brief introduction to epigenetics
 For a long time, biologists used to say that our body was made up of billions of cells, all different but all with the same genetic code. This was of course DNA, a molecule in the shape of a very long ribbon wrapped in a complex way in 23 pairs of chromosomes and which “unfolded » would be two meters long. In the traditional view, everything was at stake at the time of conception. After this, the cells divided very many times and became specialized, but keeping the same code, the same DNA. In principle, therefore, nothing changed before the creation of reproductive cells.
For a long time, biologists used to say that our body was made up of billions of cells, all different but all with the same genetic code. This was of course DNA, a molecule in the shape of a very long ribbon wrapped in a complex way in 23 pairs of chromosomes and which “unfolded » would be two meters long. In the traditional view, everything was at stake at the time of conception. After this, the cells divided very many times and became specialized, but keeping the same code, the same DNA. In principle, therefore, nothing changed before the creation of reproductive cells.
But this understanding of the fundamental code of living things has been refined. We now know that from time to time, the DNA of the cells that make up our body changes either spontaneously or under the impact of external circumstances and that even identical twins (monozygotic) do not have exactly the same genetic heritage. As we advance in age and when external circumstances are unfavorable, these changes are more and more significant. Cells have means of repairing damaged DNA, but the ability to repair appears to be reduced for people in poor health.
To this already very complex vision, we must add the dimension of a phenomenon that was still almost unknown twenty years ago: epigenetics (from the Greek « epí », « above »). This refers to mechanisms that modify gene expression without changing the nucleic acid sequence (DNA).
It is epigenetics that makes it possible to explain in particular that, while all the cells of a multicellular organism have (almost) the same genetic heritage, they develop in a totally different way according to the category of cells to which they belong, so that a skin cell « knows » that it must not develop as a heart cell.
So what exactly are epigenetic modifications?
Epigenetics is not about the transformation of DNA as such (which is the genetic code), but rather it is about changes that also take place in the cell nucleus and are closely linked to DNA. Some of these changes acquired during life can be passed on to subsequent generations, for example as a result of trauma, contrary to the principle, that was previously thought to be hard and fast, that only DNA determines how the offspring will be.
Epigenetic alterations include three mechanisms called DNA methylation, histone modifications and chromatin remodeling.
DNA methylation conditions the expression of genes in each cell. Nucleotide bases can be modified by the addition of a methyl group. This DNA modification is carried out by specific enzymes called DNMTs (for « DNA methyl-transferase »).
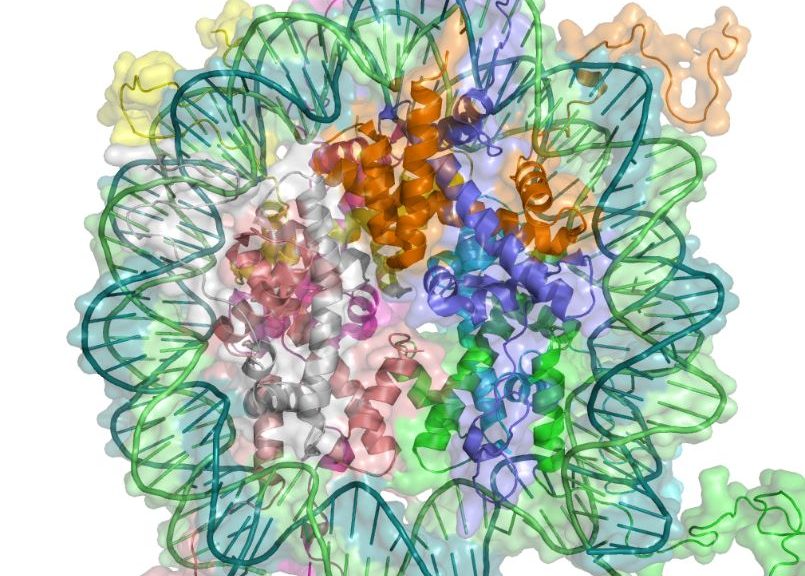
Histones are proteins that allow DNA to become compacted. Through this mechanism, DNA is wrapped around the histones like wire around a coil.
Chromatin is the material composed in particular of RNA and proteins in which DNA is parceled up and compacted, a little, here again, like a ball of yarn, but much more complex. The most « parceled up » parts have the least contact with the outside and the genes located there express themselves less or not at all. Chromatin remodeling, is the modification of this « packaging ».
The above is highly simplified. It is fascinating and vertiginous to think that each of the tens of thousands of billions of cell nuclei in our body constitutes a small universe. Each central element of the basic unit of our body, therefore, contains not only almost everything necessary for the creation of a human being, but also components organizing the expression of genetic heritage, subtle and yet indispensable differences and countless mechanisms that we still only very partially understand.
Epigenetics and measuring aging
Just as it is now getting easier and easier to decipher DNA code including differences between different cells (e.g. genetic characteristics of cancer cells), it is also easier and easier to measure differences in epigenetic components.
These components vary with the passage of time, which is why the expression epigenetic clock is used.
But if the variations of the components were only proportional to chronological age, measuring the results of this clock would not be of interest for the calculation of aging.
In fact, it turned out that the speed with which the clock advances was strongly correlated with other aging mechanisms. An aged person (or indeed an aged mouse) in poorer health will have more epigenetic changes.
It, therefore, seems possible, simply by examining at regular intervals the number of epigenetic changes in a human being’s cells, to get an idea of the speed of his or her aging.
More specifically, since epigenetic modifications are multiple mechanisms, there are many components that can be measured. These include those measured by California professor Steve Horwath and those measured by another American scientist Gregory Hannum.
As in many areas related to the causes and consequences of senescence, there is no consensus as to whether epigenetic changes are first and foremost a cause or a consequence of aging. According to some gerontologists, epigenetic changes can be considered as the engine of growth and of the development of the body and aging as a continuation of the epigenetic program. The epigenetic clock, a good predictor of causes of death, would therefore not only be one biomarker among others, but it would also be an important cause of aging, if not the most important.
A scientist from the East Coast of the United States, Josh Mitteldorf, has the ambitious project to measure thousands of epigenetic profiles of volunteer citizens undergoing anti-aging treatments of all kinds in all parts of the world over a two-year period. This project is specifically designed to look for combinations that work well together, that interact in a highly positive way. In two years, provided Josh finds funding, we could have a global vision of the effectiveness of hundreds of anti-aging treatments.
This would be immensely useful, to limit research in directions that prove to be ineffective and above all to intensify research into what works to enable millions of citizens advancing in age to take adequate preventive and curative treatment.
The good news of the month: French public project for longevity
A project called ExtenSanté is currently being examined in France by citizens, decision-makers, and scientists to promote research and treatments to combat age-related diseases. The campaign already underway includes a text explaining the approach (let’s work on causes rather than consequences), background information provided by different groups, and posters.
For more information:
Image source in.wikipedia.org/wiki/Epigenetics#/media/File:Nucleosome_1KX5_2.png. DNA associated with histone proteins to form chromatin